CONCLUSION
Alpha (α)- and beta (β)-thalassemia are inherited red blood cell disorders with a wide spectrum of symptoms, functional manifestations, and disease burden. The standard of care for α- and -β-thalassemia major is regular transfusions and iron chelation therapy. However, symptoms may persist despite treatment. Patients with non-transfusion dependent (NTD) thalassemia are historically considered to have less severe disease than patients who are transfusion-dependent (TD), yet they may experience considerable disease burden negatively affecting their health-related quality of life (HRQoL). Additionally, little is known about the HRQoL of α- thalassemia patients, of whom a majority are NTD.
This qualitative research study sought to understand the patient perspective of disease burden experienced with these disorders and explore any differences that might be related to genotype of thalassemia (α or β) or transfusion requirements.
Twenty-six adult participants (13 α, 13 β) who have made no changes to their thalassemia treatment for at least 6 months were interviewed about symptoms, impact, quality of life, and transfusion-dependence in a cross-sectional, non-interventional qualitative study. There were 8 males and 18 females; 18 were TD (5 α and 13 β) and 8 were NTD (all α). Symptoms and their impact were elicited via open-ended, semi-structured interviews. Symptom severity and bothersomeness were rated by participants on a 0-10 numerical rating scale (NRS) with 0 meaning no severity or bothersomeness, and 10 meaning extreme severity or bothersomeness. Frequency of participants reporting each symptom during the interview were calculated as a percent of the total participants in each group. Mean symptom severity and bothersomeness among participants reporting the symptom were calculated from responses to the 0-10 NRS. The results were then presented for α- and β-thalassemia, and for TD and NTD groupings of the 26 participants.All participants provided informed consent and all interviews were audio recorded and transcribed for analysis.
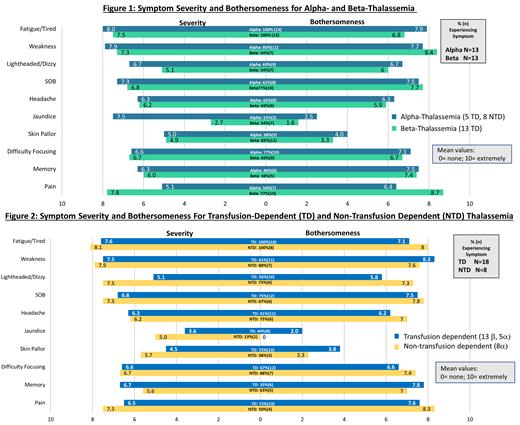
The most frequently reported symptoms among all participants were fatigue (100%), weakness (73%), shortness of breath (69%) and difficulty focusing (73%). Mean severity and bothersomeness scores for these 4 symptoms were generally similar across genotype and transfusion status, with mean severity scores for all participants ranging from 6.6 to 7.8, and mean bothersomeness scores ranging from 6.9 to 8.0 (Figures 1 and 2). Pain was reported by all groups but more frequently by TD than NTD (72% vs 50%) and β- compared to α-thalassemia participants (77% vs 54%). Pain bothersome ratings ranged from 6.4 to 8.7 indicating many of these patients are bothered or distressed by the symptom. Other frequently described and bothersome symptoms included difficulty with focused attention and memory (6 to 7.5 across all subgroups), as well as headaches (>6 across all subgroups)
Nearly half of the participants (12/26, 46%) reported considerable impact on their function and daily activities regardless of transfusion status or genotype, and a majority (14/26, 54%) reported having to make lifestyle changes because of their thalassemia. The most frequently reported limitations were physical activities, along with interference in sport and leisure activities. More than half of participants (18/26, 69%) reported emotional challenges (including being stigmatized) and having difficulty getting quality sleep.
Patients with α-NTDT and α-TD reported a similar degree of symptoms and impact on their HRQoL as patients with β-TDT, despite a historical perception of α-thalassemia as a more benign disease. The fact that many symptoms were commonly reported and were similarly severe and bothersome in both NTDT and TDT, and in both α- and β-thalassemia, suggests that patients with thalassemia are negatively impacted by their disease, regardless of transfusion dependency and genotype. Although the small sample size of this qualitative study precludes any statistical inference, the findings describe the patient experience of thalassemia and helps to inform the development of patient-relevant endpoints for future clinical trials. Additional research is needed to explore the relationship between these symptoms/HRQoL impact and clinical/pathophysiologic correlates.
Disclosures
Sheth:Bluebird bio: Consultancy, Other: Travel support; Bristol Myers Squibb/ Celegene: Consultancy, Other: Travel support, Research Funding; Agios: Consultancy, Other: Travel support, Research Funding; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Fulcrum: Consultancy; CRISPR: Membership on an entity's Board of Directors or advisory committees; Chiesi: Consultancy. Glaros:Bausch: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees. Martin:Agios: Research Funding. Abel:Agios: Consultancy, Research Funding. Lenderking:Agios: Consultancy, Research Funding; Thermo Fischer: Current Employment. Morris:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Gilroy:Agios: Current Employment, Current equity holder in publicly-traded company. Kuo:Bioverativ/Sanofi/Sangamo: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria; Vertex Pharmaceuticals: Consultancy; Pfizer: Consultancy; Novo/Nordisk: Consultancy, Honoraria; Forma Therapeutics: Consultancy; Alexion Pharmaceuticals: Consultancy; Agios Pharmaceuticals: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal